ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäļŖö ļŗ╣ļć©ļ¦Øļ¦ēļ│æņ”Ø ļŗżņØīņ£╝ļĪ£ ļæÉ ļ▓łņ¦ĖļĪ£ ĒØöĒĢ£ ļ¦Øļ¦ēĒśłĻ┤Ćņ¦łĒÖśņØ┤ļŗż.
1 ĒÅÉņćä ļČĆņ£äņŚÉ ļö░ļØ╝ ļ¦Øļ¦ēņżæņŗ¼ņĀĢļ¦źĒÅÉņćä ļ░Å ļ¦Øļ¦ēļČäņ¦ĆņĀĢļ¦źĒÅÉņćäļĪ£ ļéśļēśļ®░ Ēøäņ×ÉņØś Ļ▓ĮņÜ░ ņĀäņ×ÉņŚÉ ļ╣äĒĢśņŚ¼ ņĢĮ 5ļ░░Ļ░Ćļ¤ē ĒśĖļ░£ĒĢśļ®░ ļæÉ ņ¦łĒÖś ļ¬©ļæÉ Ļ│ĀĒśłņĢĢ, ļŗ╣ļć©, Ļ│Āņ¦ĆĒśłņ”Ø ļō▒ņØś ņĀäņŗĀņĀü ņÜöņåīļź╝ ļÅÖļ░śĒĢśĻ│Ā ņĀäņ▓┤ ņØĖĻĄ¼ņØś ņĢĮ 1%ļĪ£ ļ╣äĻĄÉņĀü ļåÆņØĆ ņ£Āļ│æļźĀņØä ļ│┤ņØĖļŗż.
2 ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņŚÉ ļÅÖļ░śļÉ£ ĒÖ®ļ░śļČĆņóģņØś Ļ▓ĮņÜ░ ņŗ£ļĀźĻ░Éņåīļź╝ ņØ╝ņ£╝ĒéżļŖö Ļ░Ćņן ņżæņÜöĒĢ£ ņøÉņØĖņ£╝ļĪ£ ĒśłĻ┤ĆņØś ĒÅÉņćäļĪ£ ņØĖĒĢ£ ņĀĢļ¦źņĢĢņØś ņ”ØĻ░ĆņŚÉ ļö░ļźĖ ĒśłļźśĒĢÖņĀüņØĖ ņÜöņØĖĻ│╝ ĒśłĻ┤Ćļé┤Ēö╝ņä▒ņןņØĖņ×É(vascular endothelial growth factor, VEGF) ļ░Å ņØĖĒä░ļŻ©Ēé© 6 (interleukin [IL]-6), ņØĖĒä░ļŻ©Ēé© 8 (IL-8) ļō▒Ļ│╝ Ļ░ÖņØĆ ņŚ╝ņ”Øņä▒ ņĀäĻĄ¼ļ¼╝ņ¦łņØś ņ”ØĻ░ĆļĪ£ ņØĖĒĢ£ ļīĆņé¼ņĀüņØĖ ņÜöņØĖņŚÉ ņØśĒĢ┤ ĒśłņĢĪļ¦Øļ¦ēņןļ▓ĮņØ┤ ĒīīĻ┤┤ļÉśļ®░ ļ░£ņāØĒĢ£ļŗż.
3 ĒÖ®ļ░śļČĆņóģņØś ņ╣śļŻīņŚÉ ņ׳ņ¢┤ ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņØś Ļ▓ĮņÜ░ ĒÖ®ļ░śļČĆ Ļ▓®ņ×É ļĀłņØ┤ņĀĆ ņ╣śļŻīņØś ĒÜ©Ļ│╝ļŖö ņĀ£ĒĢ£ņĀüņØ┤ļ®░
4 ņŚ¼ļ¤¼ ļīĆĻĘ£ļ¬© ņŚ░ĻĄ¼ļź╝ ĒåĄĒĢ┤ ņ£Āļ”¼ņ▓┤ ļé┤ ĒŖĖļ”¼ņĢöņŗ£ļåĆļĪĀ ņĢäņäĖĒåĀļéśņØ┤ļō£(triamcinolone acetonide),
5 ĒĢŁĒśłĻ┤Ćļé┤Ēö╝ņä▒ņןņØĖņ×É(anti-VEGF),
6-8 ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝(Ozurdex
®, Allergan, Irvine, CA, USA)
9,10ņØś ĒÜ©Ļ│╝ņÖĆ ņĢłņĀĢņä▒ņØ┤ ļ│┤Ļ│ĀļÉśņŚłļŗż. ļö░ļØ╝ņä£, ņØ┤ļ▓ł ņŚ░ĻĄ¼ļź╝ ĒåĄĒĢ┤ ņĀĆņ×ÉļōżņØĆ ņŗżņĀ£ ņ×äņāü ĒÖśĻ▓ĮņŚÉņä£ 1ļģäņŚ¼ņŚÉ Ļ▒Ėņ╣£ ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņŚÉ ļÅÖļ░śļÉ£ ĒÖ®ļ░śļČĆņóģ ņ╣śļŻīņŚÉ anti-VEGF ĒĢŁņ▓┤ņ╣śļŻīņĀ£ņØĖ ļ▓Āļ░öņŗ£ņŻ╝ļ¦Ö(Avastin
┬« 25 mg/mL, Genentech, San Francisco, CA, USA) ņĢłļé┤ ņŻ╝ņé¼ņÖĆ ņŖżĒģīļĪ£ņØ┤ļō£ ņĢĮļ¼╝ņØĖ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝(Ozurdex
┬«) ņé¼ņÜ® Ēøä ņØ┤ņŚÉ ļīĆĒĢ£ ĻĖ░ļŖźņĀü ļ░Å ĒĢ┤ļČĆĒĢÖņĀü ĒśĖņĀä ņĀĢļÅäļź╝ ļŗżņ¢æĒĢ£ ņ×äņāüņØĖņ×É ļ░Å Ļ▓Ćņé¼ Ļ▓░Ļ│╝ņØś ļ╣äĻĄÉļź╝ ĒåĄĒĢ┤ ņĢĮņĀ£ Ļ░äņØś ĒŖ╣ņ¦ĢņØä ĒÖĢņØĖĒĢśĻ│Āņ×É ĒĢśņśĆļŗż.
ļīĆņāüĻ│╝ ļ░®ļ▓Ģ
ļ│Ė ņŚ░ĻĄ¼ļŖö ņÜĖņé░ļīĆĒĢÖĻĄÉļ│æņøÉ ņāØļ¬ģņØśĒĢÖņŚ░ĻĄ¼ņ£żļ”¼ņŗ¼ņØśņ£äņøÉĒÜī(Institutional review board, IRB)ņŚÉņä£ Ļ▓ĆĒåĀ ļ░Å ņŖ╣ņØĖņØä ļ░øņĢśņ£╝ļ®░ (ņŖ╣ņØĖ ļ▓łĒśĖ: UUH 2022-04-036), ĒŚ¼ņŗ▒ĒéżņäĀņ¢Ė(Declaration of Helsinki)ņØś ņ£żļ”¼ ņ╣śņ╣©ņØä ņżĆņłśĒĢśņśĆļŗż. 2015ļģä 1ņøö 1ņØ╝ļČĆĒä░ 2020ļģä 12ņøö 31ņØ╝Ļ╣īņ¦Ć ļ│ĖņøÉņŚÉņä£ ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņŚÉ ļÅÖļ░śļÉ£ ĒÖ®ļ░śļČĆņóģņ£╝ļĪ£ ņ¦äļŗ© Ēøä ņ£Āļ”¼ņ▓┤ ļé┤ ņŻ╝ņé¼ņłĀņØä ņŗ£Ē¢ēļ░øĻ│Ā, ĒÖ®ļ░śļČĆņóģņØ┤ ņĢłņĀĢļÉ£ ņØ┤Ēøä 1ļģäĻ░ä ņČöņĀü Ļ┤Ćņ░░ņØ┤ Ļ░ĆļŖźĒĢśņśĆļŹś ņ┤Ø 36ļ¬ģ 36ņĢłņØä ļīĆņāüņ£╝ļĪ£ ņØśļ¼┤ĻĖ░ļĪØņØä ĒåĄĒĢ£ ĒøäĒ¢źņĀü ļČäņäØ ņŚ░ĻĄ¼ļź╝ ņŗ£Ē¢ēĒĢśņśĆļŗż. ņ£Āļ”¼ņ▓┤ ļé┤ ļ▓Āļ░öņŗ£ņŻ╝ļ¦Ö ņŻ╝ņ×ģņłĀņØä ņŗ£Ē¢ēĒĢ£ 20ņĢł, ņ£Āļ”¼ņ▓┤ ļé┤ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ ņŻ╝ņ×ģņłĀņØä ņŗ£Ē¢ēĒĢ£ 16ņĢłņØś ļæÉ ĻĄ░ņ£╝ļĪ£ ļČäļźśĒĢśņśĆĻ│Ā ļæÉ ĻĄ░ Ļ░äņØś ĒŖ╣ļ│äĒĢ£ ļČäļźś ĻĖ░ņżĆņØĆ ņŚåņŚłļŗż. ļŗ╣ļć©ļ¦Øļ¦ēļ│æņ”ØĻ│╝ Ļ░ÖņØĆ ĻĖ░ĒāĆ ļ¦Øļ¦ēĒśłĻ┤Ć ņ¦łĒÖś, ļéśņØ┤Ļ┤ĆļĀ© ĒÖ®ļ░śļ│Ćņä▒, ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņØś ņøÉņØĖņØ┤ ļÉĀ ņłś ņ׳ļŖö ļ¦Øļ¦ēĒśłĻ┤ĆņŚ╝ņØ┤ļéś ĒśłĻ┤ĆņŚ╝ņØä ņØ╝ņ£╝Ēé¼ ņłś ņ׳ļŖö ņĀäņŗĀ ņ¦łĒÖśņØ┤ ņ׳ļŖö ĒÖśņ×É, Ļ░üļ¦ē Ēś╝ĒāüņØä ĒżĒĢ©ĒĢ£ Ļ░üļ¦ē ņ¦łĒÖś, ļģ╣ļé┤ņן, ņŗ£ņČĢņØä ņ╣©ļ▓öĒĢśļŖö ļ░▒ļé┤ņן, ĒżļÅäļ¦ēņŚ╝, ļ░▒ļé┤ņןņłśņłĀņØä ņĀ£ņÖĖĒĢ£ ņĢłĻĄ¼ ļé┤ ņłśņłĀņØä ņŗ£Ē¢ēĒĢ£ ļ│æļĀź ļ░Å ņØ┤ņĀäņŚÉ ņ£Āļ”¼ņ▓┤ ļé┤ ņĢĮļ¼╝ ņŻ╝ņ×ģņłĀņØä ļ░øņØĆ ĒÖśņ×ÉļŖö ņĀ£ņÖĖĒĢśņśĆļŗż.
ņ£Āļ”¼ņ▓┤ ļé┤ ņŻ╝ņé¼ņłĀņØĆ ļŗżņØīĻ│╝ Ļ░ÖņØ┤ ņŗ£Ē¢ēĒĢśņśĆļŗż. ņŗ£ņłĀ ņĀä 0.5% proparacaine (Paracaine┬«, Hanmi Pharm., Seoul, Korea)ņ£╝ļĪ£ ņĀÉņĢłļ¦łņĘ©ĒĢ£ Ēøä 5% povidone iodineņ£╝ļĪ£ ņåŹļłłņŹ╣ņØä ĒżĒĢ©ĒĢ£ ļłł ņŻ╝ņ£äļź╝ ļŗ”ņĢśļŗż. ņØ┤Ēøä Ļ░£Ļ▓ĆĻĖ░ļź╝ ļü╝ņÜ░Ļ│Ā 5% povidone iodineĻ│╝ ņāØļ”¼ņŗØņŚ╝ņłśļĪ£ ņČ®ļČäĒ׳ ņäĖņ▓ÖĒĢ£ ļÆż ņŻ╝ņé¼ĻĖ░ņØś ļ░öļŖś ļüØņØ┤ ļłłĻ║╝ĒÆĆ Ļ░Ćņןņ×Éļ”¼ļéś ņåŹļłłņŹ╣ņŚÉ ļŗ┐ņ¦Ć ņĢŖļÅäļĪØ ņŻ╝ņØśĒĢśļ®░ 30Ļ▓īņØ┤ņ¦Ć ļ░öļŖśņØä ņØ┤ņÜ®ĒĢśņŚ¼ Ļ░üļ¦ēņ£żļČĆņŚÉņä£ 3.5 mm ļ¢©ņ¢┤ņ¦ä ņä¼ļ¬©ņ▓┤ĒÅēļ®┤ļČĆļź╝ ĒåĄĒĢ┤ ņ£Āļ”¼ņ▓┤ ļé┤ļĪ£ ļ▓Āļ░öņŗ£ņŻ╝ļ¦ÖņØä ņŻ╝ņé¼ĒĢśņśĆņ£╝ļ®░ ņ×Éņ▓┤ ņŻ╝ņ×ģ ņןņ╣śļź╝ ĒåĄĒĢ┤ ļŹ▒ņé¼ļ®öĒāĆņåÉņ×äĒöīļ×ĆĒŖĖļź╝ ņéĮņ×ģĒĢśņśĆļŗż. ņŻ╝ņ×ģ ĒøäņŚÉļŖö ļ®ĖĻĘĀ ļ®┤ļ┤ēņØä ņé¼ņÜ®ĒĢ┤ ņŻ╝ņé¼ ļČĆņ£äļź╝ ņĢĢļ░ĢĒĢśņŚ¼ ņĢĮļ¼╝ņØś ņŚŁļźśļź╝ ņśłļ░®ĒĢśņśĆĻ│Ā, ņŚŁļźśĻ░Ć ņŚåņØīņØä ĒÖĢņØĖ Ēøä moxifloxacin hydrochloride 0.5% (Vigamox┬«, Alcon Laboratories, Inc., Fort Worth, TX, USA)ļź╝ ņĀÉņĢłĒĢśĻ│Ā ņŗ£ņłĀņØä ļ¦łņ│żļŗż. ļ¬©ļōĀ ņŗ£ņłĀņØĆ ĒĢ£ ļ¬ģņØś ļ¦Øļ¦ē ņĀäļ¼ĖņØśĻ░Ć ņ¦æļÅäĒĢśņśĆļŗż.
ņØśļ¼┤ĻĖ░ļĪØņØä ĒåĄĒĢ┤ ļ¬©ļōĀ ĒÖśņ×ÉļōżņØś ņŗ£ņłĀ ņĀäņØś ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź, ņĢłņĢĢ, ņżæņŗ¼ņÖĆļæÉĻ╗śļź╝ ņĖĪņĀĢĒĢśņśĆņ£╝ļ®░ ņŗ£ņłĀ Ēøä ļ¦Øļ¦ēļé┤ļéŁĒż ļ░Å ļ¦Øļ¦ēĒĢśņĢĪņØ┤ Ļ┤Ćņ░░ļÉśņ¦Ć ņĢŖņ£╝ļ®░ ņżæņŗ¼ņÖĆļæÉĻ╗śĻ░Ć 300 ╬╝m ņØ┤ĒĢśļĪ£ Ļ░ĆļØ╝ņĢēņØĆ ņŗ£ĻĖ░ļź╝ 1ņ░© ņĢłņĀĢĻĖ░ļĪ£ ņĀĢņØśĒĢśņŚ¼ ļÅäļŗ¼ĒĢśĻĖ░Ļ╣īņ¦ĆņØś ņŻ╝ņé¼ Ēܤņłś ļ░Å ĻĖ░Ļ░ä, 1ņ░© ņĢłņĀĢĻĖ░ņØś ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź, ņĢłņĢĢ, ņżæņŗ¼ņÖĆļæÉĻ╗śļź╝ ņĖĪņĀĢĒĢśņśĆĻ│Ā ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£äņØś ļ®┤ņĀüņØä Ēæ£ņĖĄ(superficial capillary plexus)Ļ│╝ ņŗ¼ļČĆņĖĄ(deep capillary plexus)ņ£╝ļĪ£ ļéśļłäņ¢┤ ņĖĪņĀĢĒĢśņśĆļŗż. ņżæņŗ¼ņÖĆļæÉĻ╗śļŖö swept-source optical coherence tomography (SS-OCT) (DRI OCT-1 Atlantis, Topcon Corp., Tokyo, Japan)ņØś ņé╝ņ░©ņøÉ Ļ┤æĻ░ü ņ┤¼ņśü ņśüņāüņØä ņØ┤ņÜ®ĒĢśņŚ¼ 9 Early Treatment Diabetic Retinopathy Study (ETDRS) subfield 1 mm ņżæņŗ¼ņøÉņØś ļæÉĻ╗śļź╝ ņĖĪņĀĢĒĢśņśĆņ£╝ļ®░(
Fig. 1), ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀüņØś ņĖĪņĀĢņØĆ ĒīīņןĻ░Ćļ│Ć ļ╣øĻ░äņäŁļŗ©ņĖĄĒśłĻ┤ĆņĪ░ņśüņłĀ(OCT angiography; DRI OCT-1, TOPCON, Tokyo, Japan)ņØä ĒåĄĒĢ┤ ņśüņāüņØä ĒÜŹļōØĒĢ£ Ēøä ņןļ╣äņŚÉ ļé┤ņןļÉ£ ņåīĒöäĒŖĖņø©ņ¢┤ļź╝ ņé¼ņÜ®ĒĢśņśĆļŗż. IMAGEnet6 software (version 1.24, TOPCON, Tokyo, Japan)ļź╝ ņØ┤ņÜ®ĒĢśņŚ¼ ņ×ÉļÅÖņ£╝ļĪ£ ļ¦Øļ¦ēņØä ņäĖļČäĒÖöĒĢśņŚ¼ Ēæ£ņĖĄņØĆ ļé┤Ļ▓ĮĻ│äļ¦ē(inner limiting membrane)ņØś 2.6 ╬╝m ņĢäļלņŚÉņä£ ļé┤ļ¦ØņāüņĖĄ(inner plexiform layer)ņØś 15.6 ╬╝m ņĢäļלĻ╣īņ¦Ć ĒżĒĢ©ĒĢśļÅäļĪØ ĒĢśņśĆĻ│Ā, ņŗ¼ļČĆņĖĄņØĆ ļé┤ļ¦ØņāüņĖĄņØś 15.6 ╬╝m ņĢäļלņŚÉņä£ 70.2 ╬╝m ņĢäļלĻ╣īņ¦Ćļź╝ ĒżĒĢ©ĒĢśņśĆļŗż. ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£äņØś ļ®┤ņĀüņØĆ ļæÉ ļ¬ģņØś ņĖĪņĀĢņ×É(K.H.J., M.J.K.)Ļ░Ć Ļ░üĻ░ü ņłśļÅÖņ£╝ļĪ£ ļ¬©ņäĖĒśłĻ┤Ćņ┤ØņØś ņĢłņ¬Į Ļ▓ĮĻ│äļź╝ ļö░ļØ╝ ĻĘĖļ”¼ļ®┤ ņ×ÉļÅÖņ£╝ļĪ£ ļ®┤ņĀüņØ┤ ņĖĪņĀĢļÉśņŚłĻ│Ā ļæÉ Ļ░ÆņØä ĒÅēĻĘĀĻ░Æņ£╝ļĪ£ ļČäņäØĒĢśņśĆļŗż(
Fig. 2).
11,12 ņ▓½ ļ▓łņ¦Ė ņŻ╝ņé¼ Ēøä 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ņĀæņ¢┤ļōżĻĖ░Ļ╣īņ¦Ć 1Ļ░£ņøö Ļ░äĻ▓®ņ£╝ļĪ£ Ļ▓Ćņé¼ļź╝ ņŗ£Ē¢ēĒĢśļ®░ Ļ▓ĮĻ│╝ Ļ┤Ćņ░░ĒĢśņśĆņ£╝ļ®░ 1ņ░© ņĢłņĀĢĻĖ░ ņØ┤Ēøä ĒÖ®ļ░śļČĆņóģņØ┤ ņżæņŗ¼ņÖĆļæÉĻ╗ś 350 ╬╝mļź╝ ņ┤łĻ│╝ĒĢśņŚ¼ ļŗżņŗ£ ļéśĒāĆļé£ ņŗ£ĻĖ░ļź╝ ņ×¼ļ░£ļĪ£ ņĀĢņØśĒĢśņśĆļŗż.
13 ņ×¼ļ░£ ņåīĻ▓¼ņØä ļ│┤ņØĖ Ļ▓ĮņÜ░ņŚÉļŖö ņČöĻ░Ć ņŻ╝ņé¼ ņ╣śļŻīļź╝ ņŗ£Ē¢ēĒĢśņśĆņ£╝ļ®░, ņ×¼ļ░£ ņŗ£ņŚÉļŖö ņ×¼ņĢłņĀĢļÉśĻĖ░Ļ╣īņ¦Ć ļ▓Āļ░öņŗ£ņŻ╝ļ¦ÖĻĄ░ņØś Ļ▓ĮņÜ░ 1Ļ░£ņøöņØś Ļ░äĻ▓®ņ£╝ļĪ£, ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ĻĄ░ņØś Ļ▓ĮņÜ░ 3Ļ░£ņøöņØś Ļ░äĻ▓®ņ£╝ļĪ£ ņŻ╝ņé¼ļź╝ ņŗ£Ē¢ēĒĢśņśĆļŗż. ļśÉĒĢ£ 1ņ░© ņĢłņĀĢĻĖ░ ņØ┤Ēøä 1ļģä ļÆżņØś ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź, ņĢłņĢĢ, ņżæņŗ¼ņÖĆļæÉĻ╗ś, ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀüņØä ņĖĪņĀĢĒĢśņŚ¼ ļīĆņĪ░ĻĄ░ņØĖ ņĀĢņāü ļ░śļīĆņĢłĻ│╝ ļ╣äĻĄÉ ļČäņäØĒĢśņśĆļŗż. ļ¬©ļōĀ ļ╣øĻ░äņäŁļŗ©ņĖĄņ┤¼ņśü ņśüņāüņØĆ ĒĢ£ ļ¬ģņØś ļ¦Øļ¦ē ņĀäļ¼ĖņØś(M.J.K.)Ļ░Ć ļČäņäØĒĢśņśĆņ£╝ļ®░, ļ¦Øļ¦ēņĖĄņØś ĻĄ¼ļČäņØ┤ ņĀüņĀłĒĢśĻ▓ī ļéśļłäņ¢┤ņ¦ĆĻ│Ā ņśüņāüņØ┤ ņĀüĒĢ®ĒĢ£ ĒĢ┤ņāüļÅäņÖĆ ĒÆłņ¦łņØä Ļ░Ćņ¦ĆĻ│Ā ņ׳ļŖöņ¦Ć Ļ▓ĆĒåĀĒĢśņśĆļŗż.
ĒåĄĻ│ä ļČäņäØņØĆ SPSS ĒåĄĻ│ä ņåīĒöäĒŖĖņø©ņ¢┤ ĒöäļĪ£ĻĘĖļש (Version 24.0, IBM Corp, Armonk, NY, USA)ņ£╝ļĪ£ ņŗ£Ē¢ēĒĢśņśĆļŗż. Wilcoxon signed rank testņØä ņé¼ņÜ®ĒĢśņŚ¼ ņŗ£ņłĀ ņĀäĒøä ņ░©ņØ┤ļź╝ ļ╣äĻĄÉ ļČäņäØĒĢśņśĆņ£╝ļ®░, ļæÉ ĻĄ░ Ļ░äņØś ļ╣äĻĄÉļŖö Mann-Whitney U testņØä ņØ┤ņÜ®ĒĢśņśĆļŗż. ņŚ░ņåŹņĀüņØĖ ļ│ĆņłśļŖö ĒÅēĻĘĀ ┬▒ Ēæ£ņżĆĒÄĖņ░©ļĪ£ ļéśĒāĆļé┤ņŚłļŗż. pĻ░ÆņØ┤ 0.05 ļ»Ėļ¦īņØĖ Ļ▓ĮņÜ░ņŚÉ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśļŗżĻ│Ā ĒīÉļŗ©ĒĢśņśĆļŗż.
Ļ▓░ Ļ│╝
ņ┤Ø 36ļ¬ģ 36ņĢłņØś ĒÖśņ×É ņżæ ņ£Āļ”¼ņ▓┤ ļé┤ ļ▓Āļ░öņŗ£ņŻ╝ļ¦Ö ņŻ╝ņ×ģĻĄ░(intravitreal bevacizumab, IVB)ņØĆ ņ┤Ø 20ļ¬ģ 20ņĢłņØ┤ņŚłņ£╝ļ®░, ĒÅēĻĘĀ ļéśņØ┤ļŖö 64.9 ┬▒ 10.5ņäĖņśĆņ£╝ļ®░, ņ£Āļ”¼ņ▓┤ ļé┤ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ ņŻ╝ņ×ģĻĄ░(intravitreal dexamethasone, IVD)ņØĆ ņ┤Ø 16ļ¬ģ 16ņĢł, ĒÅēĻĘĀ ļéśņØ┤ļŖö 66.38 ┬▒ 8.14ņäĖņśĆļŗż. ļæÉ ĻĄ░ Ļ░äņØś ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö ņŚåņŚłļŗż(
p=0.765). ņ£äņłśņĀĢņ▓┤ņĢłņØś Ļ▓ĮņÜ░ IVBĻĄ░ņØĆ ņ┤Ø 20ņĢł ņżæ 6ņĢł, IVDĻĄ░ņØś Ļ▓ĮņÜ░ 16ņĢł ņżæ 1ņĢłņŚÉ ĒĢ┤ļŗ╣ĒĢśņśĆņ£╝ļ®░ ļæÉ ĻĄ░ Ļ░äņØś ĒåĄĻ│äņĀü ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö Ļ┤Ćņ░░ļÉśņ¦Ć ņĢŖņĢśļŗż(
p=0.236). ņ┤łĻĖ░ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØĆ IVBĻĄ░ņØ┤ 0.73 ┬▒ 0.49 logarithm of the minimal angle of resolution (logMAR), IVDĻĄ░ņØ┤ 0.82 ┬▒ 0.45 logMARņśĆņ£╝ļ®░(
p=0.386), ņżæņŗ¼ņÖĆļæÉĻ╗śļŖö Ļ░üĻ░ü 493.75 ┬▒ 150.20 ╬╝m, 479.88 ┬▒ 138.46 ╬╝m ņĖĪņĀĢļÉśņŚłļŗż(
p=0.912). ņĀäņ▓┤ ĒÖśņ×É ņżæ Ļ│ĀĒśłņĢĢņØä ņ¦Ćļŗī ĒÖśņ×ÉļŖö ņĀäņ▓┤ņØś 55.6%, ļŗ╣ļć©ļŖö 27.8%ļź╝ ņ░©ņ¦ĆĒĢśņśĆņ£╝ļ®░, ņłśņĀĢņ▓┤ņĢłņØĆ 80.6%ņŚÉ ĒĢ┤ļŗ╣ĒĢśņśĆļŗż. ņ┤łņ¦ä ņŗ£ Ļ│ĀĒśłņĢĢ, ļŗ╣ļć©, Ļ│Āņ¦ĆĒśłņ”Ø, ņä▒ļ╣ä, ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćä ņ£ĀĒśĢ, ņżæņŗ¼ņÖĆļæÉĻ╗ś, ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź ļō▒ņØĆ ĒåĄĻ│äĒĢÖņĀüņ£╝ļĪ£ ļæÉ ĻĄ░ Ļ░äņØś ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśļŗż(
Table 1).
ļ╣øĻ░äņäŁļŗ©ņĖĄņ┤¼ņśüņØä ņØ┤ņÜ®ĒĢśņŚ¼ ņĖĪņĀĢĒĢ£ ņżæņŗ¼ņÖĆļæÉĻ╗śļŖö ņŻ╝ņ×ģņłĀ ņĀäņŚÉ ļ╣äĒĢśņŚ¼ ņŻ╝ņ×ģņłĀ Ēøä 1ņ░© ņĢłņĀĢĻĖ░, 1ņ░© ņĢłņĀĢĻĖ░ ņØ┤Ēøä 1ļģä ļÆżĻ╣īņ¦Ć ļæÉ ĻĄ░ņŚÉņä£ ļ¬©ļæÉ ņØśļ»Ė ņ׳Ļ▓ī Ļ░ÉņåīĒĢśņśĆļŗż(
p<0.001). 1ņ░© ņĢłņĀĢĻĖ░ņØś ĒÅēĻĘĀ ņżæņŗ¼ņÖĆļæÉĻ╗śļŖö IVBĻĄ░ 241.95 ┬▒ 26.22 ╬╝m, IVDĻĄ░ 245 ┬▒ 43.10 ╬╝mņ£╝ļĪ£ ņ¢æ ĻĄ░ Ļ░äņŚÉ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö ņŚåņŚłļŗż(
p=0.863). 1ņ░© ņĢłņĀĢĻĖ░ 1ļģä Ļ▓ĮĻ│╝ ĒøäņØś ĒÅēĻĘĀ ņżæņŗ¼ņÖĆļæÉĻ╗śļŖö IVBĻĄ░ 255.8 ┬▒ 55.42 ╬╝m, IVDĻĄ░ 269.42 ┬▒ 55.27 ╬╝mņ£╝ļĪ£ ļæÉ ĻĄ░ Ļ░äņØś ĒåĄĻ│äĒĢÖņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö ņŚåņŚłņ£╝ļéś(
p=0.569) 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļ╣äĒĢśņŚ¼ Ļ▓Įļ»ĖĒĢśĻ▓ī ļæÉĻ╗śĻ░Ć ņ”ØĻ░ĆĒĢśļŖö Ļ▓ĮĒ¢źņØä ļ│┤ņśĆļŗż. ņ×¼ļ░£ ņŗ£ņØś ņżæņŗ¼ņÖĆļæÉĻ╗śļź╝ ļ╣äĻĄÉĒĢśņśĆņØä ļĢī IVBĻĄ░ņØś Ļ▓ĮņÜ░ ĒÅēĻĘĀ 367.07 ┬▒ 115.44 ╬╝m, IVDĻĄ░ņØĆ ĒÅēĻĘĀ 358.03 ┬▒ 115.58 ╬╝mļĪ£ ņĖĪņĀĢļÉśņŚłņ£╝ļ®░ ļæÉ ĻĄ░ Ļ░äņØś ĒåĄĻ│äĒĢÖņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö Ļ┤Ćņ░░ļÉśņ¦Ć ņĢŖņĢśļŗż(
p=0.720). ļśÉĒĢ£ IVBĻĄ░ņØś Ļ▓ĮņÜ░ 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢśĻĖ░Ļ╣īņ¦Ć ĒÅēĻĘĀ 1.95 ┬▒ 1.05ĒÜīņØś ņŻ╝ņ×ģņłĀņØ┤ ĒĢäņÜöĒĢśņśĆņ£╝ļéś IVDĻĄ░ņØś Ļ▓ĮņÜ░ IVBĻĄ░Ļ│╝ ļ╣äĻĄÉĒĢśņŚ¼ ĒÅēĻĘĀ 1.00 ┬▒ 0.00ĒÜīņØś ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśļ»ĖĒĢśĻ▓ī ņĀüņØĆ Ēܤņłśļ¦īņ£╝ļĪ£ļÅä(
p=0.004) 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢĀ ņłś ņ׳ņŚłļŗż. 1ņ░© ņĢłņĀĢĻĖ░Ļ╣īņ¦Ć ļÅäļŗ¼ĒĢśļŖö ļŹ░ņŚÉ Ļ▒Ėļ”░ ĻĖ░Ļ░äņØĆ IVBĻĄ░ņØś Ļ▓ĮņÜ░ ĒÅēĻĘĀ 2.13 ┬▒ 1.78Ļ░£ņøö, IVDĻĄ░ņØĆ 1.19 ┬▒ 0.64Ļ░£ņøöļĪ£ ļæÉ ĻĄ░ Ļ░äņØś ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö Ļ┤Ćņ░░ļÉśņ¦Ć ņĢŖņĢśļŗż(
p=0.290). 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢ£ ņØ┤Ēøä ņ×¼ļ░£ĒĢśĻĖ░ ņĀäĻ╣īņ¦ĆņØś ņĢłņĀĢ ĻĖ░Ļ░äņØä ļ╣äĻĄÉĒĢśņśĆņØä Ļ▓ĮņÜ░ņŚÉļÅä IVBĻĄ░ņØĆ ĒÅēĻĘĀ 8.37 ┬▒ 5.86Ļ░£ņøö, IVDĻĄ░ņØĆ 5.0 ┬▒ 2.80Ļ░£ņøöļĪ£ ļæÉ ĻĄ░ņŚÉņä£ ļ╣äņŖĘĒĢ£ Ļ▓ĮĒ¢źņØä ļ│┤ņśĆņ£╝ļ®░(
p=0.102) 1ņ░© ņĢłņĀĢĻĖ░ ņØ┤Ēøä 1ļģä ļÆżĻ╣īņ¦Ć ņןĻĖ░ņĀüņ£╝ļĪ£ņØś ņŻ╝ņé¼ Ēܤņłśļź╝ ļ╣äĻĄÉĒĢśņśĆņØä Ļ▓ĮņÜ░ņŚÉļÅä IVBĻĄ░ņØĆ ĒÅēĻĘĀ 2.80 ┬▒ 1.23ĒÜī, IVDĻĄ░ņØĆ ĒÅēĻĘĀ 2.44 ┬▒ 1.03ĒÜīļĪ£ ņŻ╝ņé¼ ĒܤņłśņŚÉ ņ׳ņ¢┤ņä£ ļæÉ ĻĄ░ Ļ░äņØś ĒåĄĻ│äņĀü ņ£ĀņØśļ»ĖĒĢ©ņØä ļéśĒāĆļé┤ņ¦ĆļŖö ņĢäļŗłĒĢśņśĆļŗż(
p=0.386). ņĢłņĀĢĻĖ░ņŚÉ ņĀæņ¢┤ļōĀ ņØ┤Ēøä 1ļģä ļÅÖņĢł ņĖĪņĀĢĒĢ£ ņ×¼ļ░£ Ēܤņłś ļśÉĒĢ£ IVBĻĄ░ņØĆ ĒÅēĻĘĀ 1.10 ┬▒ 1.16ĒÜī, IVDĻĄ░ņØĆ ĒÅēĻĘĀ 1.44 ┬▒ 1.03ĒÜīļĪ£ IVDĻĄ░Ļ│╝ IVBĻĄ░ņØ┤ ļ╣äņŖĘĒĢ£ ņ×¼ļ░£ Ēܤņłśļź╝ ļ│┤ņŚ¼ņŻ╝ņŚłļŗż(
p=0.352) (
Table 2).
Ļ░ü ņ╣śļŻīĻĄ░ņŚÉņä£ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØä ļ╣äĻĄÉĒĢ£ Ļ▓░Ļ│╝, 1ņ░© ņĢłņĀĢĻĖ░ ņØ┤Ēøä ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØĆ logMAR ņŗ£ļĀźņ£╝ļĪ£ IVBĻĄ░ņŚÉņä£ ĒÅēĻĘĀ 0.22 ┬▒ 0.22, IVDĻĄ░ņŚÉņä£ ĒÅēĻĘĀ 0.39 ┬▒ 0.27ņ£╝ļĪ£, ļæÉ ĻĄ░ ļ¬©ļæÉņŚÉņä£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņŗ£ļĀź ĒśĖņĀäņØä ļ│┤ņśĆņ£╝ļ®░(
p<0.001,
p=0.001) ļæÉ ĻĄ░ ņé¼ņØ┤ņŚÉņä£ļÅä IVBĻĄ░ņŚÉņä£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ļŹö ņ£ĀņØśĒĢ£ ņŗ£ļĀź ĒśĖņĀäņØä ļ│┤ņśĆļŗż(
p=0.042). 1ņ░© ņĢłņĀĢĻĖ░ 1ļģä ņØ┤Ēøä ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØĆ logMAR ņŗ£ļĀźņ£╝ļĪ£ IVBĻĄ░ņŚÉņä£ 0.22 ┬▒ 0.23, IVDĻĄ░ņŚÉņä£ 0.50 ┬▒ 0.35ņ£╝ļĪ£, 1ņ░© ņĢłņĀĢĻĖ░ņÖĆ ļÅÖņØ╝ĒĢśĻ▓ī ļæÉ ĻĄ░ ļ¬©ļæÉņŚÉņä£ ņŗ£ņłĀ ņĀä ĒÅēĻĘĀ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźĻ│╝ ļ╣äĻĄÉĒĢśņŚ¼ ņ£ĀņØśĒĢ£ ņŗ£ļĀź ĒśĖņĀäņØä ļ│┤ņśĆņ£╝ļ®░(
p<0.001,
p=0.037), ļæÉ ĻĄ░ Ļ░äņŚÉļÅä IVBĻĄ░ņŚÉņä£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ļŹö ņ£ĀņØśĒĢ£ ņŗ£ļĀź ĒśĖņĀä Ļ▓░Ļ│╝ļź╝ ļ│┤ņśĆļŗż(
p=0.007). ĒĢśņ¦Ćļ¦ī, ņ╣śļŻī ņĀäĒøä ņŗ£ļĀź ĒśĖņĀä ņĀĢļÅäļź╝ 1ņ░© ņĢłņĀĢĻĖ░ ļ░Å 1ņ░© ņĢłņĀĢĻĖ░ 1ļģä ņØ┤ĒøäņØś ņŗ£ļĀźĻ│╝ ņ╣śļŻī ņĀä ņŗ£ļĀźĻ│╝ņØś ņ░©ņØ┤ļź╝ ļČäņäØĒĢ£ Ļ▓░Ļ│╝ļŖö ļæÉ ĻĄ░ ņé¼ņØ┤ņŚÉ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśļŗż(
p=0.789,
p=0.521) (
Table 3).
Ļ░ü ĻĄ░ņŚÉņä£ ņĢłņĢĢņØä ļ╣äĻĄÉĒĢ£ Ļ▓░Ļ│╝ ņŻ╝ņ×ģņłĀ ņĀä ņĢłņĢĢņØĆ ļæÉ ĻĄ░Ļ░äņØś ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśņ£╝ļ®░(
p=0.814), 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ IVDĻĄ░ņØ┤ IVBĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśĻ▓ī ļåÆņØīņØä ļ│┤ņŚ¼ņŻ╝ņŚłņ£╝ļéś(
p=0.008), ņØ┤Ēøä 1ļģä ļÆżņŚÉļŖö ļæÉ ĻĄ░ Ļ░äņØś ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśļŗż(
p=0.369). ļæÉ ĻĄ░ ļ¬©ļæÉ ņŻ╝ņ×ģņłĀ ņĀäĻ│╝ ļ╣äĻĄÉĒĢśņŚ¼ 1ņ░© ņĢłņĀĢĻĖ░ ļ░Å ņØ┤Ēøä 1ļģä ļÆżņØś ļæÉ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ ņ£ĀņØśĒĢ£ ļ│ĆĒÖöļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśļŗż(
Table 4).
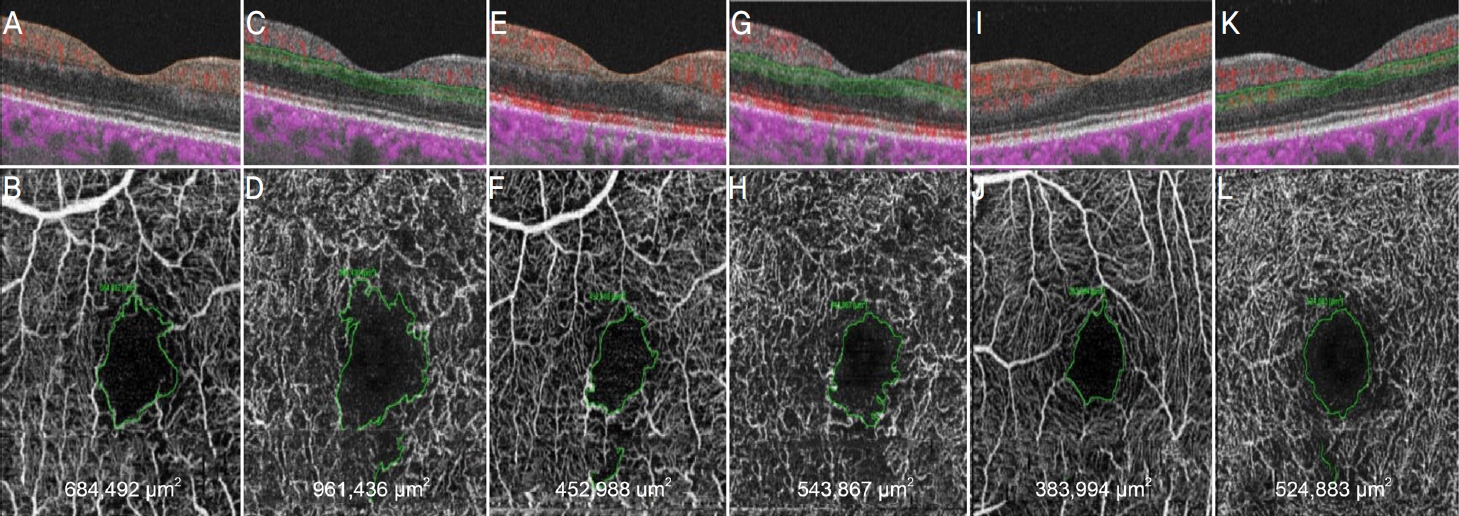
ļ╣øĻ░äņäŁļŗ©ņĖĄĒśłĻ┤ĆņĪ░ņśüņłĀņØä ņØ┤ņÜ®ĒĢśņŚ¼ ņĖĪņĀĢĒĢ£ ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀüņØś Ļ▓ĮņÜ░ Ēæ£ņĖĄĻ│╝ ņŗ¼ļČĆņĖĄņ£╝ļĪ£ Ļ░üĻ░ü ļéśļłäņ¢┤ ļ░śļīĆņĢłņØä ņĖĪņĀĢĒĢśņśĆļŗż. ņ£Āļ”¼ņ▓┤ ļé┤ ņŻ╝ņé¼ņłĀ ņØ┤Ēøä 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ņĖĪņĀĢĒĢ£ Ēæ£ņĖĄ ļ®┤ņĀüņØĆ IVBĻĄ░ņŚÉņä£ 0.548 ┬▒ 0.237 mm
2, IVDĻĄ░ņŚÉņä£ 0.529 ┬▒ 0.381 mm
2 ņĖĪņĀĢļÉśņŚłņ£╝ļ®░ ņØ┤Ēøä 1ļģä ļÆż ņ×¼ņĖĪņĀĢĒĢ£ Ļ▓░Ļ│╝ IVBĻĄ░ņØĆ 0.473 ┬▒ 0.217 mm
2, IVDĻĄ░ņØĆ 0.503 ┬▒ 0.209 mm
2ļĪ£ ņĖĪņĀĢļÉśņŚłļŗż. ļśÉĒĢ£ ņ¦łĒÖś ļ░£ļ│æ ņĀä ņāüĒā£ļź╝ ņČöņĀĢĒĢśĻĖ░ ņ£äĒĢ┤ ņĀĢņāü ļ░śļīĆņĢłņØś ļ®┤ņĀüņØä ņĖĪņĀĢĒĢśņśĆņ£╝ļ®░ IVBĻĄ░ņŚÉņä£ļŖö 0.357 ┬▒ 0.136 mm
2, IVDĻĄ░ņŚÉņä£ļŖö 0.391 ┬▒ 0.118 mm
2ļĪ£ ņĖĪņĀĢļÉśņŚłļŗż. IVDĻĄ░ņØś Ļ▓ĮņÜ░ ļæÉ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ ņ£ĀņØśĒĢ£ ļ│ĆĒÖöļź╝ ļéśĒāĆļé┤ņ¦Ć ņĢŖĻ│Ā ņĢłņĀĢņĀüņØ┤ņŚłņ£╝ļéś IVBĻĄ░ņØś Ļ▓ĮņÜ░ ņĀĢņāü ļ░śļīĆņĢłĻ│╝ ļ╣äĻĄÉĒĢśņŚ¼ 1ņ░© ņĢłņĀĢĻĖ░ ņŗ£ĻĖ░ņŚÉ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśĻ▓ī ņ”ØĻ░ĆĒĢśļŖö ņ¢æņāüņØä ļ│┤ņśĆņ£╝ļ®░(
p=0.013), ņØ┤Ēøä 1ļģä ļÆżņŚÉļŖö 1ņ░© ņĢłņĀĢĻĖ░ņÖĆ ļ╣äĻĄÉĒĢśņŚ¼ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśņ¦ä ņĢŖņ£╝ļéś Ļ░ÉņåīĒĢśņśĆļŗżĻ│Ā ĒīÉļŗ©ĒĢĀ ņłś ņ׳ņØä ņĀĢļÅäņØś ņåīĻ▓¼ņØä ļ│┤ņśĆļŗż(
p=0.052). ņäĖ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ ļæÉ ĻĄ░ Ļ░äņØś ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśļŗż(
p=0.527,
p=0.432,
p=0.705) (
Table 5). ņŗ¼ļČĆņĖĄ ņśżļ¬®ļ¼┤ĒśłĻ┤Ć ļČĆņ£ä ļ®┤ņĀüņØś Ļ▓ĮņÜ░ ņ£Āļ”¼ņ▓┤ ļé┤ ņŻ╝ņé¼ņłĀ ņØ┤Ēøä 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ņĖĪņĀĢĒĢ£ ļ®┤ņĀüņØĆ IVBĻĄ░ņŚÉņä£ 1.213 ┬▒ 0.559 mm
2, IVDĻĄ░ņŚÉņä£ 0.857 ┬▒ 0.609 mm
2ļĪ£ ņĖĪņĀĢļÉśņŚłņ£╝ļ®░ ņØ┤Ēøä 1ļģä ļÆż ņ×¼ņĖĪņĀĢĒĢ£ Ļ▓░Ļ│╝Ļ░ÆņØĆ IVBĻĄ░ņØ┤ 0.757 ┬▒ 0.465 mm
2, IVDĻĄ░ņØ┤ 0.775 ┬▒ 0.371 mm
2ļĪ£ ņĖĪņĀĢļÉśņŚłļŗż. ņĀĢņāü ļ░śļīĆņĢłņØś Ļ▓ĮņÜ░ļŖö IVBĻĄ░ņŚÉņä£ 0.667 ┬▒ 0.245 mm
2, IVDĻĄ░ņŚÉņä£ 0.608 ┬▒ 0.285 mm
2ļĪ£ ņĖĪņĀĢļÉśņŚłļŗż. IVBĻĄ░ņØś Ļ▓ĮņÜ░ Ēæ£ņĖĄ ļ®┤ņĀü ņĖĪņĀĢņŚÉņä£ņÖĆ ļÅÖņØ╝ĒĢśĻ▓ī 1ņ░© ņĢłņĀĢĻĖ░ņŚÉņä£ņØś ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ”ØĻ░Ć(
p=0.005) ņåīĻ▓¼Ļ│╝ ĒĢ©Ļ╗ś ņØ┤Ēøä 1ļģä ļÆżņŚÉņä£ļŖö ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ Ļ░Éņåī ņåīĻ▓¼ņØä ļ│┤ņśĆļŗż(
p=0.028). IVDĻĄ░ņØś Ļ▓ĮņÜ░ 1ņ░© ņĢłņĀĢĻĖ░ņŚÉņä£ ņĀĢņāü ļ░śļīĆņĢłĻ│╝ ļ╣äĻĄÉĒĢśņŚ¼ ļ╣äņŖĘĒĢ£ ņåīĻ▓¼ņØä ļ│┤ņśĆņ£╝ļ®░(
p=0.093) ņØ┤Ēøä 1ļģä ļÆżņŚÉļÅä 1ņ░© ņĢłņĀĢĻĖ░ņÖĆ ļ╣äņŖĘĒĢ£ ņåīĻ▓¼ņØä ļéśĒāĆļé┤ņŚłļŗż(
p=0.433). ņäĖ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ ļæÉ ĻĄ░ Ļ░äņØś ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļŖö ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśļŗż(
p=0.595,
p=0.053,
p=0.781). ĒŖ╣ņØ┤ņĀÉņ£╝ļĪ£ļŖö IVDĻĄ░ņØś Ļ▓ĮņÜ░ Ēæ£ņĖĄ ļ░Å ņŗ¼ļČĆņĖĄ ļ®┤ņĀüņØ┤ 1ņ░© ņĢłņĀĢĻĖ░ ļ░Å 1ļģä ļÆżņŚÉļÅä ļ╣äņŖĘĒĢśĻ▓ī ņĢłņĀĢņĀüņ£╝ļĪ£ ņ£Āņ¦ĆļÉśņŚłņ£╝ļéś IVBĻĄ░ņØś Ļ▓ĮņÜ░ Ēæ£ņĖĄ ļ░Å ņŗ¼ļČĆņĖĄ ļ¬©ļæÉņŚÉņä£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśĻ▓ī ņ”ØĻ░ĆĒĢ£ ļÆż Ļ░ÉņåīĒĢśļŖö ņ¢æņāüņØä ļ│┤ņśĆļŗżļŖö ņĀÉņØ┤ļŗż(
Table 5). ņŚ░ĻĄ¼ ĻĖ░Ļ░ä ņżæ ļæÉ ĻĄ░ ļ¬©ļæÉņŚÉņä£ ņ£Āļ”¼ņ▓┤ ļé┤ ņŻ╝ņé¼ņÖĆ ņŚ░Ļ┤ĆļÉ£ ņĢłļé┤ņŚ╝, ļģ╣ļé┤ņן, ļ¦Øļ¦ēļ░Ģļ”¼ ļō▒ņØś ņŗ¼Ļ░üĒĢ£ ĒĢ®ļ│æņ”Ø, ļČĆņ×æņÜ® ļō▒ņØĆ ļéśĒāĆļéśņ¦Ć ņĢŖņĢśļŗż.
Ļ│Ā ņ░░
ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņŚÉ ļÅÖļ░śļÉ£ ĒÖ®ļ░śļČĆņóģņØĆ ļ░£ļ│æ ĻĖ░ņĀäņØ┤ ļ│Ąņ×ĪĒĢśļ®░, ĒÖśņ×ÉņØś ļ░®ņłś ļ░Å ņ£Āļ”¼ņ▓┤ ļé┤ņŚÉļŖö ĒśłĻ┤Ćļé┤Ēö╝ņä▒ņןņØĖņ×Éļ┐Éļ¦ī ņĢäļŗłļØ╝ ļŗżņ¢æĒĢ£ inflammatory cytokine, angiopoietin-2, platelet-derived growth factor-AA, transforming growth factor-╬▓1, matrix metalloproteinase, soluble intercellular adhesion molecule ļō▒ņØ┤ ņ”ØĻ░ĆļÉśņ¢┤ ņ׳ņØīņØ┤ ļ│┤Ļ│ĀļÉ£ ļ░ö ņ׳ļŗż.
14-18 ņØ┤ļ¤¼ĒĢ£ ĒÖ®ļ░śļČĆņóģņØä ņ╣śļŻīĒĢśļŖö ļŹ░ ņ׳ņ¢┤ ņŚ¼ļ¤¼ ņŚ░ĻĄ¼ļōżņØä ĒåĄĒĢ┤ ļŗżņ¢æĒĢ£ ļ░®ļ▓ĢņØ┤ ļ░£Ēæ£ļÉśņŚłĻ│Ā, Ēśäņ×¼ļŖö ņ£Āļ”¼ņ▓┤ ļé┤ ĒĢŁĒśłĻ┤Ćļé┤Ēö╝ņä▒ņןņØĖņ×ÉņÖĆ ņŖżĒģīļĪ£ņØ┤ļō£Ļ░Ć ņŻ╝ļÉ£ ņ╣śļŻī ļ░®ļ▓ĢņŚÉ ņåŹĒĢśļéś ņäĀĒāØņŚÉ ņ׳ņ¢┤ņä£ ļ¬ģĒÖĢĒĢ£ ĻĖ░ņżĆņØ┤ ņĀĢĒĢ┤ņĀĖ ņ׳ņ¦Ć ņĢŖņØĆ ņāüĒÖ®ņØ┤ļŗż.
ļ│Ė ņŚ░ĻĄ¼ņŚÉņä£ļŖö ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņŚÉņä£ ĒÖ®ļ░śļČĆņóģņØ┤ ļÅÖļ░śļÉ£ ĒÖśņ×ÉĻĄ░ņŚÉņä£ ņ£Āļ”¼ņ▓┤ ļé┤ ļ▓Āļ░öņŗ£ņŻ╝ļ¦Ö ņŻ╝ņ×ģņłĀ ļ░Å ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ ņŻ╝ņ×ģņłĀņØä ņŗ£Ē¢ēĒĢ£ ļÆż ņéČņØś ņ¦łņŚÉ ņśüĒ¢źņØä ņżä ņłś ņ׳ļŖö ņÜöņØĖņØĖ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźĻ│╝ ĒĢ©Ļ╗ś ņĢłņĢĢ, ņżæņŗ¼ņÖĆļæÉĻ╗ś, ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀü ņĖĪņĀĢ Ēøä ļæÉ ĻĄ░ņØś ļ╣äĻĄÉļź╝ ĒåĄĒĢśņŚ¼ ĻĖ░ļŖźņĀü ļ░Å ĒĢ┤ļČĆĒĢÖņĀü Ļ▓░Ļ│╝ļź╝ ļČäņäØĒĢśņśĆļŗż. ļæÉ ĻĄ░ ļ¬©ļæÉņŚÉņä£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØś ĒśĖņĀä ļ░Å ņżæņŗ¼ņÖĆļæÉĻ╗śņØś Ļ░Éņåīļź╝ ļ│┤ņśĆļŗż. ņżæņŗ¼ņÖĆļæÉĻ╗śņØś Ļ▓ĮņÜ░ 1ņ░© ņĢłņĀĢĻĖ░ ļ░Å 1ņ░© ņĢłņĀĢĻĖ░ Ēøä 1ļģä ļÆż, ļæÉ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ ļæÉ ĻĄ░ Ļ░äņØś ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśņ£╝ļ®░ ņĀĢņāü ļ░śļīĆņĢłĻ│╝ ļ╣äĻĄÉĒĢśņŚ¼ ļæÉ ĻĄ░ ļ¬©ļæÉņŚÉņä£ ņ£ĀņØśĒĢ£ Ļ░Éņåī ņåīĻ▓¼ņØä Ļ┤Ćņ░░ĒĢĀ ņłś ņ׳ņŚłļŗż. 1ņ░© ņĢłņĀĢĻĖ░Ļ╣īņ¦Ć ļÅäļŗ¼ĒĢśļŖö ļŹ░ņŚÉ IVBĻĄ░ņØś Ļ▓ĮņÜ░ IVDĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ ļŹö ņ×”ņØĆ ņŻ╝ņé¼ ĒܤņłśĻ░Ć ĒĢäņÜöĒĢśņśĆņ£╝ļéś 1ņ░© ņĢłņĀĢĻĖ░Ļ╣īņ¦Ć ļÅäļŗ¼ĒĢśļŖö ļŹ░ņŚÉ Ļ▒Ėļ”░ ĻĖ░Ļ░äņØĆ ļæÉ ĻĄ░ņŚÉņä£ ļ╣äņŖĘĒĢ£ Ļ▓ĮĒ¢źņØä ļ│┤ņśĆļŗż. 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢ£ ļÆż ņ×¼ļ░£ĒĢśĻĖ░Ļ╣īņ¦Ć ņĢłņĀĢļÉ£ ĻĖ░Ļ░äņØĆ IVBĻĄ░Ļ│╝ IVDĻĄ░ņŚÉņä£ ļ╣äņŖĘĒĢśņśĆņ£╝ļ®░, ņ×¼ļ░£ĒĢśņśĆņØä ņŗ£ ļæÉ ĻĄ░ Ļ░äņØś ņżæņŗ¼ņÖĆļæÉĻ╗śļź╝ ļ╣äĻĄÉĒĢśņŚ¼ļÅä ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļéśĒāĆļé┤ņ¦Ć ņĢŖņĢśļŗż(
p=0.720). ļśÉĒĢ£ 1ņ░© ņĢłņĀĢĻĖ░ ņØ┤Ēøä 1ļģä ļÆżĻ╣īņ¦ĆņØś ņ×¼ļ░£ Ēܤņłś ļ░Å ņןĻĖ░ņĀüņ£╝ļĪ£ņØś ĒĢäņÜöĒĢ£ ņŻ╝ņé¼ Ēܤņłśļź╝ ņĖĪņĀĢĒĢśņŚ¼ ļ╣äĻĄÉĒĢśņśĆĻ│Ā ņ×¼ļ░£ ĒܤņłśņŚÉ ņ׳ņ¢┤ņä£ IVBĻĄ░Ļ│╝ IVDĻĄ░ņØ┤ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśņ£╝ļ®░ 1ļģä ļÆżĻ╣īņ¦ĆņØś ņןĻĖ░ņĀüņ£╝ļĪ£ ĒĢäņÜöĒĢ£ ņŻ╝ņé¼ ĒܤņłśņŚÉ ņ׳ņ¢┤ņä£ļÅä IVB, IVD ļæÉ ĻĄ░ Ļ░äņŚÉ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśļŗż(
p=0.386) (
Table 2).
ņŖżĒģīļĪ£ņØ┤ļō£ļŖö ĒĢŁĒśłĻ┤Ćļé┤Ēö╝ņäĖĒżņāØņä▒ņØĖņ×Éļź╝ Ļ░Éņåīņŗ£Ēé¼ ļ┐Éļ¦ī ņĢäļŗłļØ╝ ņŚ╝ņ”Øņä▒ ņé¼ņØ┤ĒåĀņ╣┤ņØĖņØś Ļ░ÉņåīņÖĆ ĒĢŁņŚ╝ņ”Ø ņ×æņÜ®ņØ┤ ņČöĻ░ĆļĪ£ ņ׳ņ¢┤ ņŚ╝ņ”ØņäĖĒżņØś ņØ┤ļÅÖņØä ļ¦ēņĢä ĒśłĻ┤Ć Ēł¼Ļ│╝ņä▒ņØä ņżäņØ┤ļ®░, ļČĆņóģņØä ļ░£ņāØņŗ£ĒéżļŖö ņÜöņØĖļōżņØä ļ│ĄĒĢ®ņĀüņ£╝ļĪ£ ņĪ░ņĀłĒĢśņŚ¼ ļČĆņóģņØä Ļ░Éņåīņŗ£ĒéżļŖö ĒÜ©Ļ│╝ļź╝ Ļ░ĆņĀĖņś©ļŗż. Ēśäņ×¼ ņĢłĻĄ¼ ļé┤ ņŖżĒģīļĪ£ņØ┤ļō£ ņŻ╝ņé¼ļĪ£ļŖö ĒŖĖļ”¼ņĢöņŗ£ļåĆļĪĀĻ│╝ ļŹ▒ņé¼ļ®öĒāĆņåÉņØ┤ ņé¼ņÜ®ļÉśĻ│Ā ņ׳ņ£╝ļ®░
19,20 ļŹ▒ņé¼ļ®öĒāĆņåÉņØĆ ņ¦¦ņØĆ ļ░śĻ░ÉĻĖ░ļź╝ ņ¦Ćļŗī ĒŖĖļ”¼ņĢöņŗ£ļåĆļĪĀņŚÉ ļ╣äĒĢśņŚ¼ ņĢĮ 6Ļ░£ņøöņØś ĻĖ┤ ļ░śĻ░ÉĻĖ░ļź╝ Ļ░Ćņ¦Ćļ®░, ļČĆņ×æņÜ®ņØ┤ ņĀüņ¢┤ ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņÖĆ ļŗ╣ļć©ņŚÉ ņØśĒĢ£ ĒÖ®ļ░śļČĆņóģ, ļ╣äĻ░ÉņŚ╝ņä▒ ĒøäļČĆĒżļÅäļ¦ēņŚ╝ņØś ņ╣śļŻī ļō▒ņŚÉ ļŗżņ¢æĒĢśĻ▓ī ņØ┤ņÜ®ļÉśĻ│Ā ņ׳ļŗż.
21,22 ņØ┤ļ¤¼ĒĢ£ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ņØś ĒÜ©Ļ│╝ļŖö ņĄ£ļīĆ ņŻ╝ņé¼ Ēøä 1ļŗ¼ņŚÉņä£ 3ļŗ¼Ļ╣īņ¦Ć ņ¦ĆņåŹļÉśļ®░ Pacella et al
23ņØĆ 4Ļ░£ņøö ņĀĢļÅä ņ£ĀņØśĒĢ£ ĒÜ©Ļ│╝ļź╝ ļ│┤ņØĖļŗżĻ│Ā ĒĢśņśĆļŗż. Chiquet et al
24ņØĆ ĒÖ®ļ░śļČĆņóģņØ┤ ļÅÖļ░śļÉ£ ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćä ĒÖśņ×ÉņŚÉņä£ ņןĻĖ░ņĀüņØĖ ņŗ£ļĀźņĀü, ĒĢ┤ļČĆĒĢÖņĀü Ļ▓░Ļ│╝ļź╝ Ļ│ĀļĀżĒĢśņśĆņØä ļĢīļŖö ĒĢŁĒśłĻ┤Ćļé┤ Ēö╝ņä▒ņןņØĖņ×É ņ╣śļŻīĻĄ░Ļ│╝ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ĻĄ░Ļ│╝ņØś ņ░©ņØ┤Ļ░Ć ņŚåņŚłņ£╝ļéś ļŗ©ĻĖ░ņĀüņØĖ ĻĖ░Ļ░äņŚÉ ņŗ£ļĀź ĒÜīļ│ĄņØä Ļ│ĀļĀżĒĢśņśĆņØä Ļ▓ĮņÜ░ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ĻĄ░ņØ┤ ļŹö ņÜ░ņäĖĒĢśņśĆņØīņØä ļ░£Ēæ£ĒĢśņśĆļŗż. Guignier et al
25 ļśÉĒĢ£ ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäļĪ£ ņØĖĒĢ£ ĒÖ®ļ░śļČĆņóģ ĒÖśņ×ÉņŚÉņä£ 4Ļ░£ņøö Ļ░äĻ▓®ņ£╝ļĪ£ ĒĢäņÜöņŗ£ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ ņ×¼ņ╣śļŻīļź╝ ņŗ£Ē¢ēĒĢśņśĆņØä Ļ▓ĮņÜ░ 6Ļ░£ņøöņØś ņןĻĖ░Ļ░äņØś Ļ▓ĮĻ│╝ Ļ┤Ćņ░░ ņŗ£ņŚÉļŖö ņ░©ņØ┤Ļ░Ć ņŚåņŚłņ£╝ļéś ļŗ©ĻĖ░ņĀüņ£╝ļĪ£ļŖö ļ▓Āļ░öņŗ£ņŻ╝ļ¦ÖĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ ļŹö ļ╣ĀļźĖ ņŗ£ļĀź ĒÜīļ│Ą ļ░Å ĒĢ┤ļČĆĒĢÖņĀü ĻĄ¼ņĪ░ ĒÜīļ│ĄņØä ļ│┤ņśĆļŗżĻ│Ā ņĢīļ”░ ļ░ö ņ׳ļŗż. ļ│Ė ņŚ░ĻĄ¼ņØś Ļ▓░Ļ│╝ņŚÉņä£ļÅä IVBĻĄ░Ļ│╝ ļ╣äĻĄÉĒĢśņŚ¼ IVDĻĄ░ņŚÉņä£ ĒÅēĻĘĀ 1.00 ┬▒ 0.00ĒÜīņØś ņŻ╝ņé¼ Ēܤņłśļ¦īņ£╝ļĪ£ 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢĀ ņłś ņ׳ņŚłņ£╝ļ®░ 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢśĻĖ░Ļ╣īņ¦Ć IVBĻĄ░Ļ│╝ ļ╣äĻĄÉĒĢśņŚ¼ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśļ»ĖĒĢśņ¦ĆļŖö ņĢŖņĢśņ£╝ļéś ņāüļīĆņĀüņ£╝ļĪ£ IVDĻĄ░ņŚÉņä£ 1.19 ┬▒ 0.64Ļ░£ņøöņØś ņ¦¦ņØĆ ĻĖ░Ļ░äņØ┤ ņåīņÜöļÉśņŚłļŗż. 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢ£ ļÆż ļæÉ ĻĄ░ Ļ░äņØś ņżæņŗ¼ņÖĆļæÉĻ╗śņŚÉņä£ļÅä ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤Ļ░Ć Ļ┤Ćņ░░ļÉśņ¦Ć ņĢŖņĢśļŗż. ņŖżĒģīļĪ£ņØ┤ļō£ņØś Ļ▓ĮņÜ░ ņØ┤ņĀä ņŚ░ĻĄ¼ļōżņŚÉņä£ ļŗżņ¢æĒĢ£ ļ╣łļÅäļĪ£ ņĢłņĢĢņāüņŖ╣ņØś ļČĆņ×æņÜ®ņØä ļ│┤Ļ│ĀĒĢ£ ļ░ö ņ׳ņ£╝ļ®░,
5,9,10 ļ│Ė ņŚ░ĻĄ¼ņŚÉņä£ IVB, IVDĻĄ░ņØś Ļ▓ĮņÜ░ ņŻ╝ņ×ģņłĀ ņĀäĒøäņŚÉņä£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦ä ņĢŖņĢśņ£╝ļéś ņŻ╝ņ×ģņłĀ Ēøä 1ņ░© ņĢłņĀĢĻĖ░ņŚÉņä£ IVDĻĄ░ņØ┤ IVBĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśĻ▓ī ļåÆņØĆ ņĢłņĢĢ ņłśņ╣śļź╝ ļéśĒāĆļé┤ņŚłļŗż. ĻĘĖļ¤¼ļéś 1ņ░© ņĢłņĀĢĻĖ░ņØś IVDĻĄ░ņŚÉņä£ ņČöĻ░ĆņĀüņØĖ ņĢĮļ¼╝ ļ░Å ņłśņłĀņĀü ņ╣śļŻīļŖö ĒĢäņÜöĒĢśņ¦Ć ņĢŖņĢśņ£╝ļ®░ ņØ┤Ēøä 1ļģä ļÆż ļæÉ ĻĄ░ Ļ░äņØś ņĢłņĢĢ ņ░©ņØ┤ ļśÉĒĢ£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśņ¦Ć ņĢŖņĢśļŗż.
Kim et al
26ņØĆ ļ¦Øļ¦ēļČäņ¦ĆņĀĢļ¦źĒÅÉņćäņŚÉņä£ ļ░£ņāØĒĢ£ ĒÖ®ļ░śļČĆņóģ ĒÖśņ×ÉĻĄ░ņŚÉņä£ IVBĻĄ░Ļ│╝ IVDĻĄ░ņØä ļ╣äĻĄÉĒĢśņśĆņ£╝ļ®░ ņןĻĖ░ņĀüņ£╝ļĪ£ 12Ļ░£ņøöĻ░ä Ļ┤Ćņ░░ĒĢśņśĆņØä Ļ▓ĮņÜ░ logMAR ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź ļ░Å ņżæņŗ¼ņÖĆļæÉĻ╗śņŚÉņä£ ļæÉ ĻĄ░ Ļ░äņØś Ēü░ ņ░©ņØ┤ļŖö ņŚåņŚłņ£╝ļéś 6Ļ░£ņøöĻ░äņØś Ļ┤Ćņ░░ ņŗ£ņŚÉ IVDĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ IVBĻĄ░ņŚÉņä£ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØś ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ĒśĖņĀäĻ│╝ ĒĢ©Ļ╗ś ņżæņŗ¼ņÖĆļæÉĻ╗śņØś Ļ░ÉņåīĻ░Ć Ļ┤Ćņ░░ļÉśņŚłņØīņØä ļ░£Ēæ£ĒĢ£ ļ░ö ņ׳ļŗż. Hoerauf et al
27ņØĆ ļ¦Øļ¦ēņżæņŗ¼ņĀĢļ¦źĒÅÉņćäņŚÉņä£ ļ░£ņāØĒĢ£ ĒÖ®ļ░śļČĆņóģ ĒÖśņ×Éļź╝ ļīĆņāüņ£╝ļĪ£ ĒĢ£ ļØ╝ļŗłļ╣äņŻ╝ļ¦Ö(Ranibizumab; Lucentis
┬«, Novartis AG, Basel, Switzerland)Ļ│╝ ļŹ▒ņé¼ļ®öĒāĆņåÉ ņéĮņ×ģļ¼╝ ļ╣äĻĄÉ ņŚ░ĻĄ¼ņŚÉņä£ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ĻĄ░ņŚÉņä£ļŖö 3Ļ░£ņøö ņØ┤ĒøäļĪ£ ĒÜ©Ļ│╝Ļ░Ć Ļ░ÉņåīĒĢ£ ļŹ░ņŚÉ ļ╣äĒĢśņŚ¼ ļØ╝ļŗłļ╣äņŻ╝ļ¦ÖĻĄ░ņŚÉņä£ ņĢĮļ¼╝ņØś ĒÜ©Ļ│╝Ļ░Ć ņśżļל ņ£Āņ¦ĆļÉśņŚłņ£╝ļ®░ 3Ļ░£ņøö ņØ┤ņĀäņŚÉļŖö ņ£ĀņØśĒĢ£ ņ░©ņØ┤Ļ░Ć ņŚåņŚłņ£╝ļéś 3Ļ░£ņøö ņØ┤Ēøä ļ░Å 6Ļ░£ņøö ņŗ£ņĀÉ ļ¬©ļæÉņŚÉņä£ ļŹ▒ņé¼ļ®öĒāĆņåÉ ņéĮņ×ģļ¼╝ĻĄ░ļ│┤ļŗż ņ£ĀņØśĒĢ£ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØś ĒśĖņĀäņØä ļ│┤ņśĆņØīņØä ļ░ØĒ×ī ļ░ö ņ׳ļŗż. ļ│Ė ņŚ░ĻĄ¼ņŚÉņä£ļŖö ļæÉ ĻĄ░ ļ¬©ļæÉ 1ņ░© ņĢłņĀĢĻĖ░ ļ░Å ņØ┤Ēøä 1ļģä ļÆż ņĖĪņĀĢĒĢ£ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź ļ░Å ņżæņŗ¼ņÖĆļæÉĻ╗śņŚÉņä£ ņ£ĀņØśĒĢ£ ĒśĖņĀäņØä ļ│┤ņśĆņ£╝ļ®░ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØś Ļ▓ĮņÜ░ ļæÉ ņŗ£ĻĖ░ ļ¬©ļæÉ IVBĻĄ░ņŚÉņä£ IVDĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņÜ░ņäĖĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņśĆļŗż. Gillies et al
28ņØĆ BEVORDEX ņŚ░ĻĄ¼ņŚÉņä£ ļŗ╣ļć©ĒÖ®ļ░śļČĆņóģņØä ļÅÖļ░śĒĢ£ ĒÖśņ×ÉņŚÉņä£ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ ņŻ╝ņ×ģ Ēøä ņČöĻ░ĆņĀüņØĖ ņżæņŗ¼ņÖĆļæÉĻ╗śņØś Ļ░ÉņåīĻ░Ć ļ░śļō£ņŗ£ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņ£╝ļĪ£ ņØ┤ņ¢┤ņ¦Ćņ¦ĆļŖö ņĢŖļŖöļŗżļŖö Ļ▓░Ļ│╝ļź╝ ļ░£Ēæ£ĒĢ£ ļ░ö ņ׳ļŗż. ņØ┤ņÖĆ ļ╣äņŖĘĒĢśĻ▓ī ļ│Ė ņŚ░ĻĄ¼ņØś ĒŖ╣ņØ┤ĒĢ£ ņĀÉņ£╝ļĪ£ļŖö ļæÉ ĻĄ░ Ļ░äņØś ņżæņŗ¼ņÖĆļæÉĻ╗śņŚÉņä£ļŖö ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļ│┤ņØ┤ņ¦Ć ņĢŖņĢśņ£╝ļéś ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź ĒśĖņĀä ņĀĢļÅäņŚÉņä£ IVBĻĄ░ņØ┤ IVDĻĄ░ļ│┤ļŗż ļæÉ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ ņ£ĀņØśĒĢ£ ĒśĖņĀä Ļ▓░Ļ│╝ļź╝ ļ│┤ņśĆļŗżļŖö ņĀÉņØ┤ļŗż. ņØ┤ļŖö ņ¢┤ļŖÉ ņĀĢļÅäļŖö ņāüĻ┤ĆĻ┤ĆĻ│äĻ░Ć ņ׳ņØä ņłś ņ׳ņ£╝ļéś ļ░śļō£ņŗ£ ņżæņŗ¼ņÖĆļæÉĻ╗śņØś ĒśĖņĀäņØ┤ ļ░śļō£ņŗ£ ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØś ĒśĖņĀä ņĀĢļÅäņÖĆļŖö ņØ╝ņ╣śĒĢśņ¦Ć ņĢŖļŖöļŗżļŖö ņĀÉņØä ļéśĒāĆļéĖļŗż.
ļ│Ė ņŚ░ĻĄ¼ņŚÉņä£ ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀüņØĆ IVDĻĄ░ņØś Ļ▓ĮņÜ░ Ēæ£ņĖĄ ļ░Å ņŗ¼ļČĆņĖĄņŚÉņä£ Ļ░ü ņŗ£ĻĖ░Ļ░äņØś ļ│ĆĒÖöĻ░Ć ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢśņ¦Ć ņĢŖņĢśņ£╝ļ®░ ņ”ØĻ░É ņåīĻ▓¼ ņŚåņØ┤ ļæÉ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ ņĀĢņāü ļ░śļīĆņĢłĻ│╝ ļ╣äņŖĘĒĢśĻ▓ī ņĢłņĀĢļÉśņ¢┤ ņ׳ļŖö ņåīĻ▓¼ņØä ļ│┤ņśĆļŗż. ĻĘĖļ¤¼ļéś IVBĻĄ░ņŚÉņä£ ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£äņØś ļ®┤ņĀüņØĆ 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ Ēæ£ņĖĄ ļ░Å ņŗ¼ļČĆņĖĄ ļ¬©ļæÉņŚÉņä£ ņĀĢņāü ļ░śļīĆņĢłĻ│╝ ļ╣äĻĄÉĒĢśņŚ¼ ņ£ĀņØśĒĢśĻ▓ī ļäōņ¢┤ņ¦ä ņåīĻ▓¼ņØä ļ│┤ņśĆņ£╝ļéś, 1ļģä ļÆżņŚÉļŖö 1ņ░© ņĢłņĀĢĻĖ░ ļīĆļ╣ä ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£äņØś ļ®┤ņĀüņØ┤ Ļ░ÉņåīĒĢśļŖö ņåīĻ▓¼ņØä ļ│┤ņśĆļŗż. Manousaridis
29ļŖö ĒĢŁĒśłĻ┤Ć ļé┤Ēö╝ņä▒ņןņØĖņ×ÉĻ░Ć ļ¦Øļ¦ēņØś Ēśłļźś ņł£ĒÖśņŚÉ ņśüĒ¢źņØä ņŻ╝ņ¢┤ ņןĻĖ░ņĀüņ£╝ļĪ£, ņ×Āņ×¼ņĀüņ£╝ļĪ£ ĒÖ®ļ░śļČĆ ĒŚłĒśłņŚÉ ĻĖ░ņŚ¼ĒĢĀ ņłś ņ׳ņ£╝ļ®░ ĒŖ╣Ē׳ļéś ņŚ¼ļ¤¼ ļ▓łņØś ļ░śļ│ĄļÉ£ ĒĢŁĒśłĻ┤Ćļé┤Ēö╝ņä▒ņןņØĖņ×É ņŻ╝ņé¼Ļ░Ć ņ׳ņØä Ļ▓ĮņÜ░ ņØ┤ļź╝ Ļ░ĆņåŹĒÖöņŗ£Ēé¼ ņłś ņ׳ņØīņØä ļ░£Ēæ£ĒĢ£ ļ░ö ņ׳ļŗż. ļ│Ė ņŚ░ĻĄ¼ņŚÉņä£ļÅä ņĢłņĀĢļÉ£ ņāüĒā£Ļ░Ć ņ£Āņ¦ĆļÉśļŖö IVDĻĄ░Ļ│╝ ļ╣äĻĄÉĒĢśņŚ¼ IVBĻĄ░ņŚÉņä£ ņ¦ĆņåŹņĀüņ£╝ļĪ£ 1ņ░© ņĢłņĀĢĻĖ░ ļ░Å ņØ┤Ēøä 1ļģä ļÆżņŚÉņä£ ĻĖēĻ▓®ĒĢ£ ņ”ØĻ░É ņåīĻ▓¼ņØä ļéśĒāĆļé┤ļŖö Ļ▓āņ£╝ļĪ£ ļ│┤ņĢä, ļ░śļ│ĄņĀüņØĖ ĒĢŁĒśłĻ┤Ćļé┤Ēö╝ņä▒ņןņØĖņ×É ņŻ╝ņé¼ ņ╣śļŻī ņ×Éņ▓┤Ļ░Ć Ēśłļźś Ļ│ĄĻĖē ļ░Å ĒśłĻ┤ĆņØś ĒĢ┤ļČĆĒĢÖņĀüņØĖ ĻĄ¼ņĪ░ņŚÉ ņśüĒ¢źņØä ņŻ╝ņŚłņØä Ļ▓āņ£╝ļĪ£ ņāØĻ░üļÉ£ļŗż.
ļ│Ė ņŚ░ĻĄ¼ņŚÉņä£ņØś ĒĢ£Ļ│äņĀÉņ£╝ļĪ£ļŖö ņÜ░ņäĀ ļ¼┤ņ×æņ£ä ļ░░ņĀĢņØ┤ ņØ┤ļŻ©ņ¢┤ņ¦ĆļŖö ņĀäĒ¢źņĀü ņŚ░ĻĄ¼ ļ░®ļ▓ĢĻ│╝ ļŗ¼ļ”¼ ņĀüņØĆ ņłśļź╝ ļīĆņāüņ£╝ļĪ£ ņØ┤ļŻ©ņ¢┤ņ¦ä ĒøäĒ¢źņĀü ņŚ░ĻĄ¼ļĪ£ņä£ ņäĀĒāØ ļ╣äļÜżļ”╝(selection bias)ņØ┤ ļ░£ņāØĒĢĀ Ļ░ĆļŖźņä▒ņØ┤ ļåÆļŗż. ļśÉĒĢ£ ņŗ£ņłĀ Ēøä ļ░Å ņŚ░ĻĄ¼ ļÅäņżæ Ļ▓ĮĻ│╝ Ļ┤Ćņ░░ ĻĖ░Ļ░äļÅÖņĢł ņŗżņŗ£ĒĢ£ ņäĖĻĘ╣ļō▒Ēśäļ»ĖĻ▓ĮĻ▓Ćņé¼ņāü ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀź ļō▒ņØś Ļ▓░Ļ│╝ņŚÉ ļÜ£ļĀĘĒĢśĻ▓ī ņśüĒ¢źņØä ņŻ╝ņŚłņØä ļ¦īĒĢ£ ļ░▒ļé┤ņןņØś ņ¦äĒ¢ē ņĀĢļÅäĻ░Ć Ļ┤Ćņ░░ļÉśņ¦ĆļŖö ņĢäļŗłĒĢśņśĆņ£╝ļéś ņĀĢļ¤ēņĀüņØ┤ņ¦Ć ļ¬╗ĒĢśĻ│Ā ņŻ╝Ļ┤ĆņĀüņØ┤ļØ╝ļŖö ĒĢ£Ļ│äĻ░Ć ņ׳ņ¢┤ ļ░▒ļé┤ņן ļ░£ņāØņ£╝ļĪ£ ņØĖĒĢ┤ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ĻĄ░ņŚÉņä£ ņŗ£ļĀź ņśłĒøä Ļ▓░Ļ│╝ ļČäņäØņŚÉ ņśüĒ¢źņØä ļ»Ėņ│żņØä ņłś ņ׳ļŗżļŖö ņĀ£ĒĢ£ņĀÉņØ┤ ņ׳ļŗż. IVBĻĄ░ņØś Ļ▓ĮņÜ░ ņ£äņłśņĀĢņ▓┤ņĢłņØś ļ╣äņ£©ņØ┤ 20ņĢł ņżæ 6ņĢł, IVDĻĄ░ņØś Ļ▓ĮņÜ░ 16ņĢł ņżæ 1ņĢłņØä ņ░©ņ¦ĆĒĢśļŖöļŹ░, ņØ┤ļĪ£ ņØĖĒĢśņŚ¼ ņ£ä ņłśņĀĢņ▓┤ņĢłņØś ļ╣äņ£©ņØ┤ ļé«ņØĆ IVDĻĄ░ņŚÉņä£ ĒÖ®ļ░śļČĆņóģņØ┤ ĒÜīļ│ĄļÉśĻ│Ā ļé£ ņØ┤ĒøäņŚÉļÅä ļ░▒ļé┤ņןņ£╝ļĪ£ ņØĖĒĢ£ ņśüĒ¢źņ£╝ļĪ£ ņŗ£ļĀź ĒÜīļ│ĄņŚÉ ņĀ£ĒĢ£ņØ┤ ņāØĻ▓© Ļ▓░Ļ│╝ņŚÉ ņśüĒ¢źņØä ņŻ╝ņŚłņØä Ļ░ĆļŖźņä▒ ļśÉĒĢ£ Ļ│ĀļĀżĒĢśņŚ¼ņĢ╝ ĒĢ£ļŗż. ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀü ņĖĪņĀĢņØś Ļ▓ĮņÜ░ ļ╣øĻ░äņäŁļŗ©ņĖĄĒśłĻ┤ĆņĪ░ņśüņłĀņØä 1ĒÜīļ¦ī ņé¼ņÜ®ĒĢśņŚ¼ ņĖĪņĀĢĒĢśņśĆņ£╝ļ®░, ļ╣äļĪØ 2ļ¬ģņØś ņĀĆņ×ÉĻ░Ć ņĖĪņĀĢĒĢ£ ļ®┤ņĀüņØä ĒÅēĻĘĀĒĢśņŚ¼ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņØśļ»Ė ņ׳ļŖö Ļ▓░Ļ│╝ļź╝ ņ¢╗ņŚłņ£╝ļéś, ņłśļÅÖņ£╝ļĪ£ ĻĘĖļĀżņä£ ņĀĢĒĢśņŚ¼ Ļ░£ņØĖņØś ņŻ╝Ļ┤ĆņØ┤ Ļ░£ņ×ģļÉĀ ņłś ņ׳ļŗżļŖö ņĀ£ĒĢ£ņĀÉ ļśÉĒĢ£ ņ׳ļŗż.
Ļ▓░ļĪĀņĀüņ£╝ļĪ£ ļ¦Øļ¦ēņĀĢļ¦źĒÅÉņćäņŚÉ ļÅÖļ░śļÉ£ ĒÖ®ļ░śļČĆņóģņØä ņ╣śļŻīĒĢśļŖö ļŹ░ ņ׳ņ¢┤ ļ▓Āļ░öņŗ£ņŻ╝ļ¦Ö ļ░Å ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ ņ╣śļŻīĻĄ░ ļ¬©ļæÉņŚÉņä£ 1ņ░© ņĢłņĀĢĻĖ░ ļ░Å ņØ┤Ēøä 1ļģäĻ╣īņ¦Ć ņןĻĖ░ņĀüņ£╝ļĪ£ ĒĢ┤ļČĆĒĢÖņĀü ļ░Å ĻĖ░ļŖźņĀüņØĖ Ļ░£ņäĀņØä ļ│┤ņŚ¼ņŻ╝ņŚłļŗż. ĻĘĖļ¤¼ļéś 1ņ░© ņĢłņĀĢĻĖ░ ļÅäļŗ¼Ļ╣īņ¦ĆņØś ņĀüņØĆ ņŻ╝ņé¼ Ēܤņłś, ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀüņØś ņĀüņØĆ ļ│ĆļÅÖņä▒ ļō▒ņØä Ļ│ĀļĀżĒĢśņśĆņØä ļĢī ņĢłņĀĢĻĖ░Ļ╣īņ¦ĆņØś ņŻ╝ņé¼ ĒܤņłśņØś ņĄ£ņåīĒÖö ļ░Å ĒĢ┤ļČĆĒĢÖņĀü ĻĄ¼ņĪ░ņØś ĒÜīļ│ĄņØ┤ ļ¬®Ēæ£ņØ╝ Ļ▓ĮņÜ░ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ņØä Ļ│ĀļĀżĒĢśņŚ¼ ļ│╝ ņłś ņ׳Ļ▓Āļŗż. IVBĻĄ░ņØś Ļ▓ĮņÜ░ IVDĻĄ░ļ│┤ļŗż 1ņ░© ņĢłņĀĢĻĖ░ņŚÉ ļÅäļŗ¼ĒĢśĻĖ░Ļ╣īņ¦ĆņØś ļ¦ÄņØĆ ņŻ╝ņé¼ Ēܤņłśļź╝ Ļ│ĀļĀżĒĢśņŚ¼ņĢ╝ ĒĢśļ®░, 1ņ░© ņĢłņĀĢĻĖ░ņŚÉņä£ņØś Ēæ£ņĖĄ ļ░Å ņŗ¼ļČĆņĖĄ ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£ä ļ®┤ņĀüņØś ņ”ØĻ░ĆņÖĆ ņØ┤Ēøä 1ļģä ļÆżņŚÉņä£ņØś Ļ░Éņåī ņåīĻ▓¼ņ£╝ļĪ£ ĒÖ®ļ░śļČĆņØś ĒŚłĒśłņØä ņĢģĒÖöņŗ£Ēé¼ ņłś ņ׳ļŖö Ļ░ĆļŖźņä▒ņØä ņŗ£ņé¼ĒĢśņśĆļŗż. ĻĘĖļ¤¼ļéś ņ¦üņĀæņĀüņØĖ ņéČņØś ņ¦łĻ│╝ ņŚ░Ļ▓░ļÉśļŖö ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØś ĒÜīļ│Ą ņĀĢļÅäĻ░Ć ļæÉ ņŗ£ĻĖ░ ļ¬©ļæÉņŚÉņä£ IVDĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ ņÜ░ņłśĒĢśņśĆļŗż. ļśÉĒĢ£ ņ¦ĆņåŹņä▒ ņĖĪļ®┤ņŚÉņä£ļÅä IVDĻĄ░Ļ│╝ ļ╣äĻĄÉĒĢśņŚ¼ ņ×¼ļ░£ ņĀäĻ╣īņ¦Ć ņĢłņĀĢ ņāüĒā£Ļ░Ć ņ£Āņ¦ĆļÉśļŖö ĻĖ░Ļ░ä, ņ×¼ļ░£ Ēܤņłś ļ░Å ņןĻĖ░ņĀüņ£╝ļĪ£ ĒĢäņÜöĒĢ£ ņŻ╝ņé¼ Ēܤņłś ļō▒ņŚÉņä£ ĒåĄĻ│äņĀüņ£╝ļĪ£ ņ£ĀņØśĒĢ£ ņ░©ņØ┤ļź╝ ļéśĒāĆļé┤ņ¦Ć ņĢŖņĢśļŗż. ļö░ļØ╝ņä£ ļ╣äņÜ®ņĀüņØĖ ņĖĪļ®┤ ļ░Å ĻĖ░ļŖźņĀüņØĖ ņĖĪļ®┤ņŚÉņä£ļŖö ņČ®ļČäĒ׳ Ļ│ĀļĀżĒĢ┤ ļ│╝ ņłś ņ׳ļŖö ņ╣śļŻīļØ╝ ņāØĻ░üļÉ£ļŗż. ņØ┤ņÖĆ ļŹöļČłņ¢┤ IVBĻĄ░ņŚÉņä£ IVDĻĄ░ņŚÉ ļ╣äĒĢśņŚ¼ ņśżļ¬®ļ¼┤ĒśłĻ┤ĆļČĆņ£äņØś ņŗ¼ļČĆņĖĄņØś ļ®┤ņĀüņØ┤ ņ”ØĻ░ĆĒĢśņśĆņØīņŚÉļÅä ņĄ£ļīĆĻĄÉņĀĢņŗ£ļĀźņØś ĒśĖņĀäņØä ļ│┤ņØ┤ļŖö ņØ╝ņ╣śĒĢśņ¦Ć ņĢŖļŖö Ļ▓░Ļ│╝ņŚÉ ļīĆĒĢśņŚ¼ ņĀäĒ¢źņĀüņØĖ ļīĆĻĘ£ļ¬©ņØś ņןĻĖ░ ņŚ░ĻĄ¼Ļ░Ć ĒĢäņÜöĒĢĀ Ļ▓āņ£╝ļĪ£ ļ│┤ņØĖļŗż. ĻĘĖļ¤¼ļéś ļ│Ė ņŚ░ĻĄ¼ļŖö ļ▓Āļ░öņŗ£ņŻ╝ļ¦ÖĻ│╝ ļŹ▒ņé¼ļ®öĒāĆņåÉņéĮņ×ģļ¼╝ņØ┤ļØ╝ļŖö ņä£ļĪ£ ļŗżļźĖ ņĢĮļÅÖĒĢÖņĀü ĻĖ░ņĀäņØä Ļ░¢Ļ│Ā ņ׳ļŖö ņĢĮņĀ£ļź╝ ļ╣äĻĄÉĒĢ£ ņŚ░ĻĄ¼ļØ╝ļŖö ņĀÉņŚÉņä£ ņØśņØśĻ░Ć ņ׳ļŗżĻ│Ā ĒĢĀ ņłś ņ׳ņ£╝ļ®░ ĒÖśņ×É Ļ░£Ļ░£ņØĖņØś ņāüĒÖ®ņØä Ļ│ĀļĀżĒĢśņŚ¼ ņ¢┤ļ¢ż ņ╣śļŻīļź╝ ņäĀĒāØĒĢĀ ņłś ņ׳ņØäņ¦ĆņŚÉ ļÅäņøĆņØ┤ ļÉĀ ņłś ņ׳ņØä Ļ▓āņ£╝ļĪ£ ņāØĻ░üļÉ£ļŗż.
Figure┬Ā1.
A 12 ├Ś 9 mm wide three-dimensional optical coherence tomography (OCT) scan was obtained from a 64-year-old female patient with macular edema related to a branch retinal vein occlusion. Total retinal thickness was measured in the nine Early Treatment Diabetic Retinopathy Study (ETDRS) subfields. OCT scan shows increased central foveal thickness (A) with multiple exudates and intraretinal cystic edema (B) at a baseline. After injection of the first intravitreal dexamethasone implant, macular edema got resolved, and central foveal thickness returned to normal range (C), (D ) and reached the first stabilization period. After 1 year, the macula was stable showing no interval change, and central foveal thickness also remained calm (E), (F), while during that period, there were no other recurrences. (G) and (H) show retinal thickness map of normal fellow eye.

Figure┬Ā2.
Optical coherence tomography (OCT) and OCT angiography (OCTA) images of a 57-year-old male patient with macular edema related to a central retinal vein occlusion. Measurements of the foveal avascular zone (FAZ) area was conducted using OCTA. En face images were generated for retinal vascular networks: superficial capillary plexus (SCP) and deep capillary plexus (DCP) at the first stable stage after intravitreal bevacizumab injection (A-D), and at the 1 year after the first stable stage (E-H), and in normal fellow eye (I-L). The FAZ area was defined as the area inside the central border of the capillary network and determined by manually outlining the inner border of foveal capillaries using the OCTA system software.
Table┬Ā1.
Baseline demographic and clinical characteristics of the study participants
|
Parameter |
IVB |
IVD |
p-value |
|
Total number of patients (eyes) |
20 |
16 |
- |
|
Age (years) |
64.90 ┬▒ 10.50 |
66.38 ┬▒ 8.14 |
0.765 |
|
Sex (male:female) |
9:11 |
6:10 |
>0.999 |
|
Lens status (phakic:pseudophakic) |
14:6 |
15:1 |
0.236 |
|
Diabetes mellitus |
5 |
5 |
0.765 |
|
Hypertension |
10 |
10 |
0.765 |
|
Hyperlipidemia |
5 |
3 |
0.765 |
|
Mean baseline CFT (╬╝m) |
493.75 ┬▒ 150.20 |
479.88 ┬▒ 138.46 |
0.912 |
|
Mean baseline BCVA (logMAR) |
0.73 ┬▒ 0.49 |
0.82 ┬▒ 0.45 |
0.386 |
|
RVO type (BRVO:CRVO) |
11:9 |
8:8 |
0.814 |
Table┬Ā2.
Comparison of central foveal thickness between the eyes with IVB and IVD, between baseline and 1st stable stage, and between baseline and 1 year after 1st stable stage
|
IVB |
IVD |
p-value*
|
|
Baseline CFT (╬╝m) |
493.75 ┬▒ 150.20 |
479.88 ┬▒ 138.46 |
0.912 |
|
1st stable stage CFT (╬╝m) |
241.95 ┬▒ 26.22 |
245.00 ┬▒ 43.10 |
0.863 |
|
p-valueŌĆĀ
|
<0.001 |
<0.001 |
|
|
Duration period (month) |
2.13 ┬▒ 1.78 |
1.19 ┬▒ 0.64 |
0.290 |
|
Injection time |
1.95 ┬▒ 1.05 |
1.0 ┬▒ 0.0 |
0.004 |
|
Relapsed CFT (╬╝m) |
367.07 ┬▒ 115.44 |
358.03 ┬▒ 115.58 |
0.720 |
|
1 year after 1st stable stage CFT (╬╝m) |
255.80 ┬▒ 55.42 |
269.42 ┬▒ 55.27 |
0.569 |
|
p-valueŌĆĪ
|
<0.001 |
0.001 |
|
|
Stable period after 1st stable stage (month) |
8.37 ┬▒ 5.86 |
5.0 ┬▒ 2.80 |
0.102 |
|
Relapse time during 1 year |
1.10 ┬▒ 1.16 |
1.44 ┬▒ 1.03 |
0.352 |
|
Total injection time |
2.80 ┬▒ 1.23 |
2.44 ┬▒ 1.03 |
0.386 |
Table┬Ā3.
Comparison of visual acuity and visual gain between the eyes with IVB and IVD, between baseline and 1st stable stage, and between baseline and 1 year after 1st stable stage
|
BCVA (logMAR) |
IVB |
IVD |
p-value*
|
|
Baseline |
0.73 ┬▒ 0.49 |
0.82 ┬▒ 0.45 |
0.386 |
|
1st stable stage |
0.22 ┬▒ 0.22 |
0.39 ┬▒ 0.27 |
0.042 |
|
p-valueŌĆĀ
|
<0.001 |
0.001 |
|
|
Visual gain |
0.51 ┬▒ 0.45 |
0.43 ┬▒ 0.35 |
0.789 |
|
1 year after 1st stable stage |
0.22 ┬▒ 0.23 |
0.50 ┬▒ 0.35 |
0.007 |
|
p-valueŌĆĪ
|
<0.001 |
0.037 |
|
|
Visual gain |
0.50 ┬▒ 0.43 |
0.31 ┬▒ 0.48 |
0.521 |
Table┬Ā4.
Comparison of intraocular pressure between the eyes with IVB and IVD, between baseline and 1st stable stage, and between baseline and 1 year after 1st stable stage
|
IOP (mmHg) |
IVB |
IVD |
p-value*
|
|
Baseline |
16.13 ┬▒ 2.16 |
16.56 ┬▒ 2.34 |
0.814 |
|
1st stable stage |
15.55 ┬▒ 2.63 |
18.44 ┬▒ 3.64 |
0.008 |
|
p-valueŌĆĀ
|
0.118 |
0.082 |
|
|
1 year after 1st stable stage |
15.25 ┬▒ 2.67 |
16.69 ┬▒ 3.70 |
0.369 |
|
p-valueŌĆĪ
|
0.081 |
0.813 |
|
Table┬Ā5.
Comparisons of foveal avascular zone areas (superficial capillary plexus and deep capillary plexus) between the eyes with IVB and IVD, between the normal fellow eye and 1st stable stage, and between the normal fellow eye and 1 year after 1st stable stage, and between the eyes with 1st stable stage and 1 year after 1st stable stage
|
FAZ area (mm2) |
IVB |
IVD |
p-value*
|
|
SCP |
|
|
|
|
ŌĆāNormal fellow eyes |
0.357 ┬▒ 0.136 |
0.391 ┬▒ 0.118 |
0.527 |
|
ŌĆā1st stable stage |
0.548 ┬▒ 0.237 |
0.529 ┬▒ 0.381 |
0.432 |
|
ŌĆāp-valueŌĆĀ
|
0.013 |
0.216 |
|
|
1 year after 1st stable stage |
0.473 ┬▒ 0.217 |
0.503 ┬▒ 0.209 |
0.705 |
|
ŌĆāp-valueŌĆĪ
|
0.071 |
0.254 |
|
|
ŌĆāp-value┬¦
|
0.052 |
0.221 |
|
|
DCP |
|
|
|
|
ŌĆāNormal fellow eyes |
0.667 ┬▒ 0.245 |
0.608 ┬▒ 0.285 |
0.595 |
|
ŌĆā1st stable stage |
1.213 ┬▒ 0.559 |
0.857 ┬▒ 0.609 |
0.053 |
|
ŌĆāp-valueŌĆĀ
|
0.005 |
0.093 |
|
|
1 year after 1st stable stage |
0.757 ┬▒ 0.465 |
0.775 ┬▒ 0.371 |
0.781 |
|
ŌĆāp-valueŌĆĪ
|
0.984 |
0.156 |
|
|
ŌĆāp-value┬¦
|
0.028 |
0.433 |
|
REFERENCES
1) Cakir M, Dogan M, Bayraktar Z, et al. Efficacy of intravitreal triamcinolone for the treatment of macular edema secondary to branch retinal vein occlusion in eyes with or without grid laser photocoagulation. Retina 2008;28:465-72.


4) The Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion: the central vein occlusion study group M report. Ophthalmology 1995;102:1425-33.

6) Zhang H, Liu ZL, Sun P, Gu F. Intravitreal bevacizumab for treatment of macular edema secondary to central retinal vein occlusion: eighteen-month results of a prospective trial. J Ocul Pharmacol Ther 2011;27:615-21.


7) Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1124-33.e1.


8) Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology 2014;121:202-8.


9) Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011;118:2453-60.


11) Min JK, Lee S, Kim JS, et al. Effects of diabetic macular edema on repeatability of retinal nerve fiber layer thickness measurements at the macular and peripapillary area using swept-source optical coherence tomography. Curr Eye Res 2017;42:307-14.


12) Woo JM, Yoon YS, Woo JE, Min JK. Foveal avascular zone area changes analyzed using OCT angiography after successful rhegmatogenous retinal detachment repair. Curr Eye Res 2018;43:674-8.


13) Patrao NV, Antao S, Egan C, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom national health service setting. Am J Ophthalmol 2016;172:51-7.


14) Kiire CA, Chong NV. Managing retinal vein occlusion. BMJ 2012;344:e499.


16) Jung SH, Kim KA, Sohn SW, Yang SJ. Association of aqueous humor cytokines with the development of retinal ischemia and recurrent macular edema in retinal vein occlusion. Invest Ophthalmol Vis Sci 2014;55:2290-6.


17) Tuuminen R, Loukovaara S. Increased intravitreal angiopoietin-2 levels in patients with retinal vein occlusion. Acta Ophthalmol 2014;92:e164-5.


19) Sohn HJ, Han DH, Lee DY, Nam DH. Changes in aqueous cytokines after intravitreal triamcinolone versus bevacizumab for macular oedema in branch retinal vein occlusion. Acta Ophthalmol 2014;92:e217-24.


20) Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin 1992;10:505-12.


21) Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121:1904-14.


22) Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117:1134-46.e3.


23) Pacella E, Vestri AR, Muscella R, et al. Preliminary results of an intravitreal dexamethasone implant (Ozurdex
®) in patients with persistent diabetic macular edema. Clin Ophthalmol 2013;7:1423-8.


26) Kim M, Lee DH, Byeon SH, et al. Comparison of intravitreal bevacizumab and dexamethasone implant for the treatment of macula oedema associated with branch retinal vein occlusion. Br J Ophthalmol 2015;99:1271-6.


27) Hoerauf H, Feltgen N, Weiss C, et al. Clinical efficacy and safety of ranibizumab versus dexamethasone for central retinal vein occlusion (COMRADE C): a European label study. Am J Ophthalmol 2016;169:258-67.


28) Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 2014;121:2473-81.


29) Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol 2012;96:179-84.


Biography
Ļ╣ĆĒśĢņŻ╝ / Hyeong Ju Kim
ņÜĖņé░ļīĆĒĢÖĻĄÉ ņØśĻ│╝ļīĆĒĢÖ ņÜĖņé░ļīĆĒĢÖĻĄÉļ│æņøÉ ņĢłĻ│╝ĒĢÖĻĄÉņŗż
Department of Ophthalmology, Ulsan University Hospital, University of Ulsan College of Medicine








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print